

 Overview
Overview The MALUCTM Total Hip Arthroplasty System (THA) is used for primary total hip replacement in skeletally mature individuals.
The system consists of monolithic cemented and press-fit femoral stems, modular CoCr and BIOLOX® delta femoral heads in various sizes and offsets, uncemented acetabular shells and conventional polyethylene liners.
Accessory components include distal centralizers, cancellous bone screws and cement restrictors. Instrumentation necessary for proper implantation is also included.
 Features
Features
Materials of Components:
Metal Femoral Head: Config Drawing No: SW-00006-14 Material = CoCrMo (casted) Instruments
Instruments  Indication
Indication
The SurgTech MALUCTM System is intended for use for cases of severely painful and/or disabled joint from osteoarthritis, traumatic arthritis, rheumatoid arthritis or congenital hip dysplasia, avascular necrosis of the femoral head, acute traumatic fracture of the femoral head or neck, failed previous hip surgery (including joint reconstruction, internal fixation, arthrodesis, hemiarthroplasty and/or total arthroplasty), and certain cases of ankylosis.
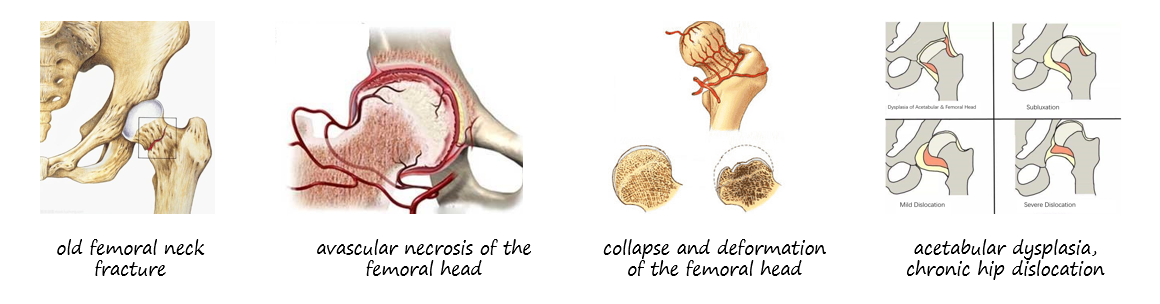
(1) patients with old femoral neck fracture, femoral head or acetabular damage and pain, which affects joint function.
(2) patients with avascular necrosis of the femoral head, collapse and deformation of the femoral head and destruction of the acetabulumContact
Tel: (440) 899-2922 E-mail: chengbao@surgtech-med.com Address: 24600 Center Ridge Road, Suite 195 Westlake OH 44145